AbbVie (NYSE: ABBV) spooked the market in early October, but the sell-off is a buying opportunity. The critical takeaway is that only the earnings guidance was altered; the trim was small and offset by the cause. The cause is an increased expectation for intellectual property, R&D, and milestone acquisitions-related expenses that aid the pipeline and growth sustainability outlook.
Analysts respond favorably to the news, with four price target revisions in the first few days after release. The latest is from Truist Financial, which pegs the stock at a Buy and raises its target to $215. The $215 is 10% above the price action in early October, aligning with a revision trend leading the market higher. All but one target issued since the Q2 report is above the consensus, nearly 90%, and suggests new highs for this stock will come soon.
AbbVie Turns a Corner in 2024
AbbVie turned a corner in 2024, walking right over the patent cliff and surviving on the strength of its diversified portfolio. The company returned to top-line growth despite the contraction in Humira sales, with sales of Skyrizi and Rinvoq driving growth in the Immunology segment. Sales of Oncology therapies grew by 10.5% and Neuroscience by 14.7% on strength in budding blockbusters like Vraylar, Imbruvica, and Venclexta. Sales growth is expected to continue due to the increasing use of these therapies, expanded approvals, and the strength of the pipeline. The analysts forecast revenue to grow by 2.5% this year, accelerate to 5% in 2025, and widen the margin.
Pipeline updates since the Q2 earnings release include positive results from a late-stage trial for Tavapadon. Tavapadon is an experimental treatment for Parkinson's Disease that reached its main goal of significantly reducing disease severity as a single agent. The current leading treatment for Parkinson’s is levodopa, which was developed early in the 20th century and is currently made by a variety of pharmaceutical companies, including AbbVie. The market for levodopa is growing and is estimated to hit $3.2 billion by 2030, so approval of Tavapadon would be a significant win.
Oncology is another catalyst for AbbVie. The recent acquisition of Immunogen and developments in the ADC portfolio have it on track to expand its solid-tumor portfolio. Updates on several promising candidates are due by the end of the year.
Institutions Buy AbbVie in 2024, Provide a Tailwind for the Market
The institutional activity is providing a tailwind for AbbVie's stock price in 2024. The institutions have bought the pharma stock on balance for seven consecutive quarters, lifting their total interest to over 70%. The buying is broad-based among institutions with significant holdings in state-run, mutual fund, ETF, and private management accounts. Their activity coincides with a 50% increase in the stock price and is likely to continue providing upward price pressure in 2025.
The reasons for institutional interest are growth, cash flow, balance sheet, and dividends. The company’s growth trajectory aligns with improving cash flow, a healthy balance sheet, and sustained capital returns and capital return growth. As it is, the dividend is worth more than $6 per share annually and yields more than 3%, with the stock trading at record levels. It is also growing at a double-digit CAGR.
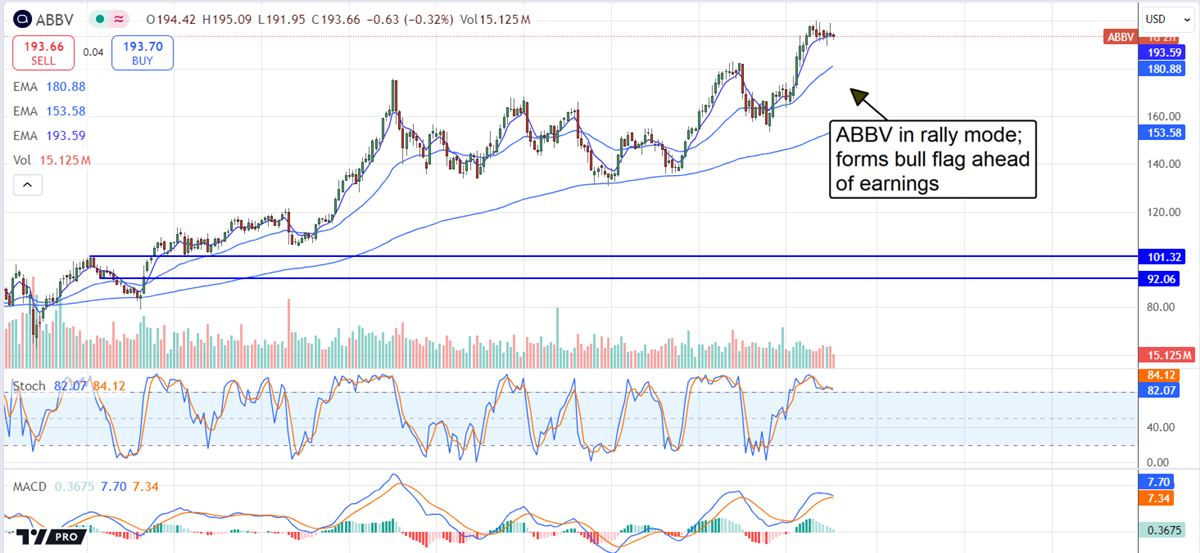
AbbVie stock is trending higher and poised to make another new high soon. The recent price pullback didn’t get very deep and set the market up to fire a continuation signal with a $35 or 20% upside potential. Price action since May is consistent with a Bullish Flag and only needs to set a new high to confirm it. In that scenario, the market will likely advance the magnitude of the flag pole, a gain of $35 or 20% of the pre-rally price.