Paragon 28, Inc. (NYSE: FNA), is pleased to announce the launch of its Phantom® Metatarsal Shortening System which features a first in-kind intramedullary device for fixation of in-line shortening osteotomies of the lesser metatarsals. These osteotomies are preformed to prevent metatarsalgia and floating toe, two of the most frequently addressed foot and ankle pathologies. The system includes a patent pending cut guide allowing for increased precision when performing the correction.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230424005048/en/
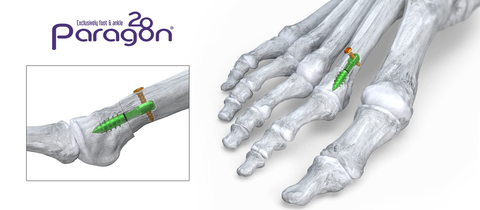
Phantom® Metatarsal Shortening System (Graphic: Business Wire)
Mark Myerson, MD, Phantom® design surgeon, said, "Many implants are re-iterations of existing products, however the Phantom® Metatarsal Shortening System is truly unique, innovative, and quite novel. The ability to precisely and accurately shorten the metatarsal with simple and rigid fixation will be a tremendous addition to the management of various forefoot pathologies".
Paragon 28’s CEO, Albert DaCosta, commented, “We set out to design an implant that addressed the limitations of current procedures, in particular the soft tissue imbalance associated with shortening procedures of the lesser metatarsals. We are thrilled to have accomplished this ambition with the launch of the Phantom® Metatarsal Shortening System and expect this addition to catalyze growth in the forefoot segment of our business. We understand the value that comes from having a comprehensive forefoot portfolio to pair with our bunion offering allowing facilities and surgeons to work with a single partner to meet all their forefoot and bunion needs.”
The Phantom® Metatarsal Shortening System bolsters Paragon 28’s forefoot and bunion solutions offering, which includes the TenoTac™, Paratrooper™, HammerTube™, Gorilla® Precision™ Lapidus Plate, and Phantom® MIS Lapidus Nail. With this comprehensive portfolio, Paragon 28® provides its customers with a single source to address their forefoot and bunion needs.
Paragon 28® is grateful for the significant contributions that Mark Myerson, MD, Jose Sanhudo, MD, and Lew Schon, MD, made in the design of this system.
About Paragon 28, Inc.
Based in Englewood, CO., Paragon 28, is a leading medical device company exclusively focused on the foot and ankle orthopedic market and is dedicated to improving patient lives. From the onset, Paragon 28® has provided innovative orthopedic solutions, procedural approaches and instrumentation that cover a wide range of foot and ankle ailments including fracture fixation, forefoot, ankle, progressive collapsing foot deformity (PCFD) or flatfoot, Charcot foot and orthobiologics. The company designs products with both the patient and surgeon in mind, with the goal of improving outcomes, reducing ailment recurrence and complication rates, and making the procedures simpler, consistent, and reproducible.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230424005048/en/
Contacts
Investor Contact:
Matt Brinckman
Senior Vice President, Strategy and Investor Relations
(720) 912-1332
mbrinckman@paragon28.com





